Cationic Polymer Nanoparticles-Mediated Delivery of miR-124 Impairs Tumorigenicity of Prostate Cancer Cells
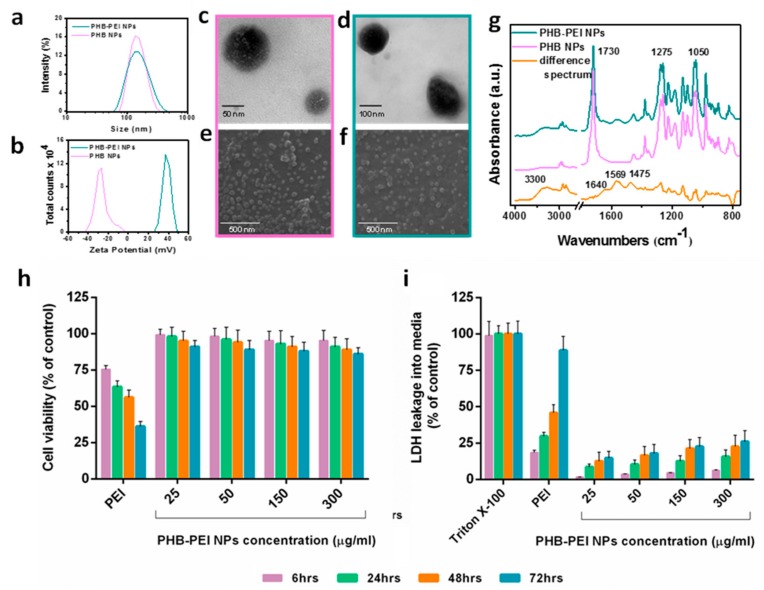
MicroRNAs (miRNAs) play a pivotal role in regulating the expression of genes involved in tumor development, invasion, and metastasis. In particular, microRNA-124 (miR-124) modulates the expression of carnitine palmitoyltransferase 1A (CPT1A) at the post-transcriptional level, impairing the ability of androgen-independent prostate cancer (PC3) cells to completely metabolize lipid substrates. However, the clinical translation of miRNAs requires the development of effective and safe delivery systems able to protect nucleic acids from degradation. Herein, biodegradable polyethyleneimine-functionalized polyhydroxybutyrate nanoparticles (PHB-PEI NPs) were prepared by aminolysis and used as cationic non-viral vectors to complex and deliver miR-124 in PC3 cells. Notably, the PHB-PEI NPs/miRNA complex effectively protected miR-124 from RNAse degradation, resulting in a 30% increase in delivery efficiency in PC3 cells compared to a commercial transfection agent (Lipofectamine RNAiMAX). Furthermore, the NPs-delivered miR-124 successfully impaired hallmarks of tumorigenicity, such as cell proliferation, motility, and colony formation, through CPT1A modulation. These results demonstrate that the use of PHB-PEI NPs represents a suitable and convenient strategy to develop novel nanomaterials with excellent biocompatibility and high transfection efficiency for cancer therapy.
Keywords: aminolysis; gene delivery; miR-124; polyethyleneimine (PEI); polyhydroxybutyrate (PHB); polymer nanoparticles; prostate cancer.
Bibliografia:
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Ca: A Cancer J. Clin. 2019, 69, 7–34,
doi:10.3322/caac.21551.
- Saad, F.; Fizazi, K. Androgen Deprivation Therapy and Secondary Hormone Therapy in the Management
of Hormone‐sensitive and Castration‐resistant Prostate Cancer. Urology 2015, 86, 852–861,
doi:10.1016/j.urology.2015.07.034.
- Nuhn, P.; De Bono, J.S.; Fizazi, K.; Freedland, S.J.; Grilli, M.; Kantoff, P.W.; Sonpavde, G.; Sternberg, C.N.;
Yegnasubramanian, S.; Antonarakis, E.S. Update on Systemic Prostate Cancer Therapies: Management of
Metastatic Castration‐resistant Prostate Cancer in the Era of Precision Oncology. Eur Urol 2019, 75, 88–99,
doi:10.1016/j.eururo.2018.03.028.
- Vanacore, D.; Boccellino, M.; Rossetti, S.; Cavaliere, C.; DʹAniello, C.; Di Franco, R.; Romano, F.J.;
Montanari, M.; La Mantia, E.; Piscitelli, R.; et al. Micrornas in prostate cancer: An overview. Oncotarget
2017, 8, 50240–50251, doi:10.18632/oncotarget.16933.
- Bryzgunova, O.E.; Konoshenko, M.Y.; Laktionov, P.P. MicroRNA‐guided gene expression in prostate
cancer: Literature and database overview. J. Gene Med. 2018, 20, e3016‐e3016, doi:10.1002/jgm.3016.
- Ni, J.; Bucci, J.; Chang, L.; Malouf, D.; Graham, P.; Li, Y. Targeting MicroRNAs in Prostate Cancer
Radiotherapy. Theranostics 2017, 7, 3243–3259, doi:10.7150/thno.19934.
- Kanwal, R.; Plaga, A.R.; Liu, X.; Shukla, G.C.; Gupta, S. MicroRNAs in prostate cancer: Functional role as
biomarkers. Cancer Lett 2017, 407, 9–20, doi:10.1016/j.canlet.2017.08.011.
- Shukla, K.K.; Misra, S.; Pareek, P.; Mishra, V.; Singhal, B.; Sharma, P. Recent scenario of microRNA as
diagnostic and prognostic biomarkers of prostate cancer. Urol. Oncol. 2017, 35, 92–101,
doi:10.1016/j.urolonc.2016.10.019.
- Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy
Prediction and Therapeutic Tools for Breast Cancer. Theranostics 2015, 5, 1122–1143, doi:10.7150/thno.11543.
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. miRNA
nanotherapeutics for cancer. Drug Discov. Today 2017, 22, 424–432, doi:10.1016/j.drudis.2016.10.014.
- Mucaj, V.; Lee, S.S.; Skuli, N.; Giannoukos, D.N.; Qiu, B.; Eisinger‐Mathason, T.S.K.; Nakazawa, M.S.; Shay,
J.E.S.; Gopal, P.P.; Venneti, S.; et al. MicroRNA‐124 expression counteracts pro‐survival stress responses in
glioblastoma. Oncogene 2015, 34, 2204–2214, doi:10.1038/onc.2014.168.
- Lee, Y.; Kim, H.J.; Park, C.K.; Kim, Y.‐G.; Lee, H.‐J.; Kim, J.‐Y.; Kim, H.‐H. MicroRNA‐124 regulates
osteoclast differentiation. Bone 2013, 56, 383–389, doi:10.1016/j.bone.2013.07.007.
- Li, L.; Luo, J.; Wang, B.; Wang, D.; Xie, X.; Yuan, L.; Guo, J.; Xi, S.; Gao, J.; Lin, X.; et al. Microrna‐124 targets
flotillin‐1 to regulate proliferation and migration in breast cancer. Mol. Cancer 2013, 12, 163–163,
doi:10.1186/1476‐4598‐12‐163.
- Zhu, J.; Wang, S.; Zhang, W.; Qiu, J.; Shan, Y.; Yang, D.; Shen, B. Screening key microRNAs for castration‐
resistant prostate cancer based on miRNA/mRNA functional synergistic network. Oncotarget 2015, 6,
43819–43830, doi:10.18632/oncotarget.6102.
- Chu, M.; Chang, Y.; Guo, Y.; Wang, N.; Cui, J.; Gao, W.‐Q. Regulation and methylation of tumor suppressor
miR‐124 by androgen receptor in prostate cancer cells. Plos ONE 2015, 10, e0116197,
doi:10.1371/journal.pone.0116197.
- Shi, X.‐B.; Ma, A.‐H.; Xue, L.; Li, M.; Nguyen, H.G.; Yang, J.C.; Tepper, C.G.; Gandour‐Edwards, R.; Evans,
C.P.; Kung, H.‐J.; et al. miR‐124 and Androgen Receptor Signaling Inhibitors Repress Prostate Cancer
Growth by Downregulating Androgen Receptor Splice Variants, EZH2, and Src. Cancer Res. 2015, 75, 5309–
5317, doi:10.1158/0008‐5472.CAN‐14‐0795.
- Shi, X.B.; Xue, L.; Ma, A.H.; Tepper, C.G.; Gandour‐Edwards, R.; Kung, H.J.; deVere White, R.W. Tumor
suppressive miR‐124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene
2013, 32, 4130–4138, doi:10.1038/onc.2012.425.
- Valentino, A.; Calarco, A.; Di Salle, A.; Finicelli, M.; Crispi, S.; Calogero, R.A.; Riccardo, F.; Sciarra, A.;
Gentilucci, A.; Galderisi, U.; et al. Deregulation of MicroRNAs mediated control of carnitine cycle in
prostate cancer: Molecular basis and pathophysiological consequences. Oncogene 2017, 36, 6030–6040,
doi:10.1038/onc.2017.216.
- Hu, J.; Sheng, Y.; Shi, J.; Yu, B.; Yu, Z.; Liao, G. Long Circulating Polymeric Nanoparticles for Gene/Drug
Delivery. Curr. Drug Metab. 2018, 19, 723–738, doi:10.2174/1389200219666171207120643.
- Fujita, Y.; Kuwano, K.; Ochiya, T. Development of small RNA delivery systems for lung cancer therapy.
Int. J. Mol. Sci. 2015, 16, 5254–5270, doi:10.3390/ijms16035254.
- Muthiah, M.; Park, I.‐K.; Cho, C.‐S. Nanoparticle‐mediated delivery of therapeutic genes: Focus on miRNA
therapeutics. Expert Opin. Drug Deliv. 2013, 10, 1259–1273, doi:10.1517/17425247.2013.798640.
- Mauri, E.; Perale, G.; Rossi, F. Nanogel Functionalization: A Versatile Approach To Meet the Challenges of
Drug and Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541, doi:10.1021/acsanm.8b01686.
- Saraiva, C.; Paiva, J.; Santos, T.; Ferreira, L.; Bernardino, L. MicroRNA‐124 loaded nanoparticles enhance
brain repair in Parkinsonʹs disease. J. Control. Release 2016, 235, 291–305, doi:10.1016/j.jconrel.2016.06.005.
- Louw, A.M.; Kolar, M.K.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Kjems, J.; Novikov, L.N. Chitosan
polyplex mediated delivery of miRNA‐124 reduces activation of microglial cells in vitro and in rat models
of spinal cord injury. Nanomedicine 2016, 12, 643–653, doi:10.1016/j.nano.2015.10.011.
- Schulze, J.; Kuhn, S.; Hendrikx, S.; Schulz‐Siegmund, M.; Polte, T.; Aigner, A. Spray‐Dried Nanoparticle‐
in‐Microparticle Delivery Systems (NiMDS) for Gene Delivery, Comprising Polyethylenimine (PEI)‐Based
Nanoparticles in a Poly(Vinyl Alcohol) Matrix. Small 2018, 14, doi:10.1002/smll.201701810.
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non‐viral vector for nucleic acid
delivery. Mater. Sci. Eng. C 2016, 68, 904–918, doi:10.1016/j.msec.2016.07.066.
- Peng, L.; Wagner, E. Polymeric Carriers for Nucleic Acid Delivery: Current Designs and Future Directions.
Biomacromolecules 2019, 20, 3613–3626, doi:10.1021/acs.biomac.9b00999.
- Shrivastav, A.; Kim, H.‐Y.; Kim, Y.‐R. Advances in the applications of polyhydroxyalkanoate nanoparticles
for novel drug delivery system. Biomed. Res. Int 2013, 2013, 581684–581684, doi:10.1155/2013/581684.
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of
natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221,
doi:10.1002/jctb.4685.
- Meng, D.‐C.; Chen, G.‐Q. Synthetic Biology of Polyhydroxyalkanoates (PHA). Adv. Biochem. Eng. Biotechnol.
2018, 162, 147–174, doi:10.1007/10_2017_3.
- Pişkin, E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995, 6, 775–795,
doi:10.1163/156856295x00175.
- Calarco, A.; Bosetti, M.; Margarucci, S.; Fusaro, L.; Nicolì, E.; Petillo, O.; Cannas, M.; Galderisi, U.; Peluso,
G. The genotoxicity of PEI‐based nanoparticles is reduced by acetylation of polyethylenimine amines in
human primary cells. Toxicol. Lett. 2013, 218, 10–17, doi:10.1016/j.toxlet.2012.12.019.
- Höbel, S.; Aigner, A. Polyethylenimines for siRNA and miRNA delivery in vivo. Wiley Interdiscip. Rev.
Nanomed. Nanobiotechnol. 2013, 5, 484–501, doi:10.1002/wnan.1228.
- dʹAyala, G.G.; Calarco, A.; Malinconico, M.; Laurienzo, P.; Petillo, O.; Torpedine, A.; Peluso, G. Cationic
copolymers nanoparticles for nonviral gene vectors: Synthesis, characterization, and application in gene
delivery. J. Biomed. Mater. Res. A 2010, 94, 619–630, doi:10.1002/jbm.a.32752.
- Moret, I.; Esteban Peris, J.; Guillem, V.M.; Benet, M.; Revert, F.; Dasí, F.; Crespo, A.; Aliño, S.F. Stability of
PEI‐DNA and DOTAP‐DNA complexes: Effect of alkaline pH, heparin and serum. J. Control. Release 2001,
76, 169–181, doi:10.1016/s0168‐3659(01)00415‐1.
- Sun, X.; Zhang, N. Cationic polymer optimization for efficient gene delivery. Mini Rev. Med. Chem. 2010,
10, 108–125, doi:10.2174/138955710791185109.
- Zhupanyn, P.; Ewe, A.; Büch, T.; Malek, A.; Rademacher, P.; Müller, C.; Reinert, A.; Jaimes, Y.; Aigner, A.
Extracellular vesicle (ECV)‐modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in
vitro and in vivo. J. Control. Release 2019, 319, 63–76, doi:10.1016/j.jconrel.2019.12.032.
- Zhou, Y.; Yu, F.; Zhang, F.; Chen, G.; Wang, K.; Sun, M.; Li, J.; Oupický, D. Cyclam‐Modified PEI for
Combined VEGF siRNA Silencing and CXCR4 Inhibition To Treat Metastatic Breast Cancer.
Biomacromolecules 2018, 19, 392–401, doi:10.1021/acs.biomac.7b01487.
- Huang, W.; Zhang, C. Tuning the Size of Poly(lactic‐co‐glycolic Acid) (PLGA) Nanoparticles Fabricated by
Nanoprecipitation. Biotechnol. J. 2018, 13, doi:10.1002/biot.201700203.
- Croll, T.I.; OʹConnor, A.J.; Stevens, G.W.; Cooper‐White, J.J. Controllable surface modification of
poly(lactic‐co‐glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical
aspects. Biomacromolecules 2004, 5, 463–473, doi:10.1021/bm0343040.
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending poly(3‐
hydroxybutyrate) with tannic acid: Influence of a polyphenolic natural additive on the rheological and
thermal behavior. Eur. Polym. J. 2015, 63, 123–131, doi:https://doi.org/10.1016/j.eurpolymj.2014.12.021.
- Lakard, B.; Herlem, G.; Lakard, S.; Antoniou, A.; Fahys, B. Urea potentiometric biosensor based on
modified electrodes with urease immobilized on polyethylenimine films. Biosens. Bioelectron. 2004, 19,
1641–1647, doi:https://doi.org/10.1016/j.bios.2003.12.035.
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine)‐mediated gene delivery affects endothelial cell
function and viability. Biomaterials 2001, 22, 471–480, doi:10.1016/s0142‐9612(00)00203‐9.
- Kunath, K.; von Harpe, A.; Fischer, D.; Petersen, H.; Bickel, U.; Voigt, K.; Kissel, T. Low‐molecular‐weight
polyethylenimine as a non‐viral vector for DNA delivery: Comparison of physicochemical properties,
transfection efficiency and in vivo distribution with high‐molecular‐weight polyethylenimine. J. Control.
Release 2003, 89, 113–125, doi:10.1016/s0168‐3659(03)00076‐2.
- Morachis, J.M.; Mahmoud, E.A.; Almutairi, A. Physical and chemical strategies for therapeutic delivery by
using polymeric nanoparticles. Pharm. Rev. 2012, 64, 505–519, doi:10.1124/pr.111.005363.
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and
characteristics governing cellular internalization and trans‐barrier trafficking of nanostructures. Int. J.
Nanomed. 2015, 10, 2191–2206, doi:10.2147/IJN.S75615.
- Devulapally, R.; Sekar, N.M.; Sekar, T.V.; Foygel, K.; Massoud, T.F.; Willmann, J.K.; Paulmurugan, R.
Polymer nanoparticles mediated codelivery of antimiR‐10b and antimiR‐21 for achieving triple negative
breast cancer therapy. ACS Nano 2015, 9, 2290–2302, doi:10.1021/nn507465d.
- Wong, L.‐Y.; Xia, B.; Wolvetang, E.; Cooper‐White, J. Targeted, Stimuli‐Responsive Delivery of Plasmid
DNA and miRNAs Using a Facile Self‐Assembled Supramolecular Nanoparticle System. Biomacromolecules
2018, 19, 353–363, doi:10.1021/acs.biomac.7b01462.
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Biophys. Acta 2013, 1831, 1518–
1532, doi:10.1016/j.bbalip.2013.03.010.
- Long, J.; Zhang, C.‐J.; Zhu, N.; Du, K.; Yin, Y.‐F.; Tan, X.; Liao, D.‐F.; Qin, L. Lipid metabolism and
carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791.
- Galbraith, L.; Leung, H.Y.; Ahmad, I. Lipid pathway deregulation in advanced prostate cancer. Pharm. Res.
2018, 131, 177–184, doi:10.1016/j.phrs.2018.02.022.
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I:
Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226, doi:10.1038/cddis.2016.132.
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system
and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 228–228, doi:10.1038/s41419‐018‐0313‐7.
- Aiderus, A.; Black, M.A.; Dunbier, A.K. Fatty acid oxidation is associated with proliferation and prognosis
in breast and other cancers. BMC Cancer 2018, 18, 805–805, doi:10.1186/s12885‐018‐4626‐9.
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020,
122, 4–22, doi:10.1038/s41416‐019‐0650‐z.
- Stoykova, G.E.; Schlaepfer, I.R. Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New
Opportunities for Therapy. Int J. Mol. Sci. 2019, 20, 2626, doi:10.3390/ijms20112626.
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijón, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.;
Glodé, L.M.; Eckel, R.H.; et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol.
Cancer 2014, 13, 2361–2371, doi:10.1158/1535‐7163.MCT‐14‐0183.
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.;
Peluso, G.; Tafuri, A. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional
preclinical effects in leukemias. Blood 2015, 126, 1925–1929, doi:10.1182/blood‐2014‐12‐617498.
- Flaig, T.W.; Salzmann‐Sullivan, M.; Su, L.‐J.; Zhang, Z.; Joshi, M.; Gijón, M.A.; Kim, J.; Arcaroli, J.J.; Van
Bokhoven, A.; Lucia, M.S.; et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen
blockade. Oncotarget 2017, 8, 56051–56065, doi:10.18632/oncotarget.17359.
- Wang, Y.‐N.; Zeng, Z.‐L.; Lu, J.; Wang, Y.; Liu, Z.‐X.; He, M.‐M.; Zhao, Q.; Wang, Z.‐X.; Li, T.; Lu, Y.‐X.; et
al. CPT1A‐mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis.
Oncogene 2018, 37, 6025–6040, doi:10.1038/s41388‐018‐0384‐z.
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial
polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–
R4,doi:https://doi.org/10.1016/0378‐5173(89)90281‐0.
